Evolving Innovation On Expression Systems
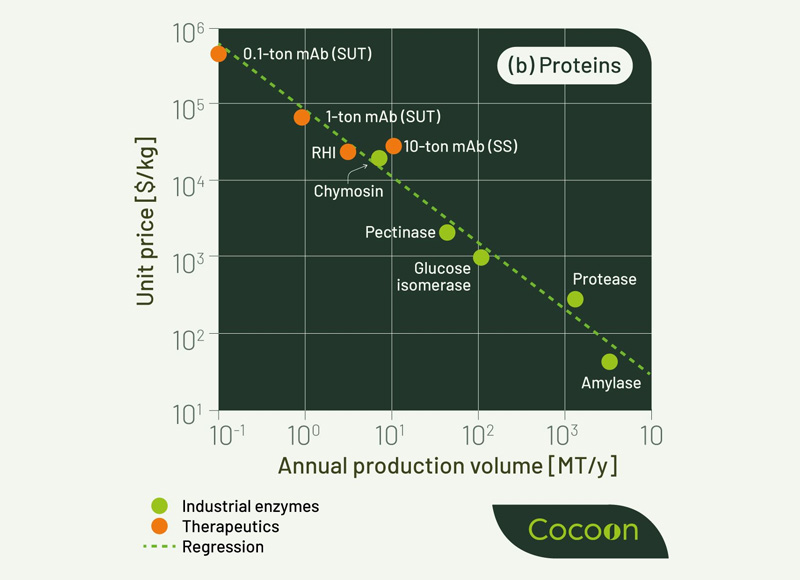
According to Humbird (2021), in a research paper published several years ago, there was a graph showing that for recombinant proteins of all kinds – enzymes, antibodies, growth factors, etc. –a high correlation exists between the scale of production and the price point on a per kilogram basis. As you might expect, price drops logarithmically as scale increases; enzymes produced at 100s of kilotons scale, like those that help make our laundry detergent better at removing stains or enzymes that aid livestock animals to digest food, can be produced at $30/kilogram or less. Antibodies used in humans, like immunotherapy to treat cancer, might only need 100 kilograms to a ton of product a year, but the average price is correspondingly higher, closer to $100 – $300/gram.
Producing these recombinant proteins at all scales is done through microbial fermentation – take an organism like bacteria, yeast, or mammalian cells, and program it to make the right product. Innovation exists throughout this industry. With advances on in silico predictions of activity using AI & Machine Learning, we can design better proteins that have higher activity; we have achieved great progress around making proteins better at what they are designed to do. Additionally, with HTP screening and Design-Build-Test-Learn cycles, we can lower the cost of protein production by making the cell more productivity – more protein is produced (output) with the same amount of culture media (input).
What I found as an interesting exercise was to take this graph and extend the x-axis out a bit – does the linear range hold true if we look at even smaller scales, for bioproduction enzymes like those involved in the production of mRNA such as T7 RNA Polymerase, with global demand at a kilogram or thereabouts? It turns out, when you start to plot out these enzymes needed at a kilogram or less, the pricing correlation breaks down; these enzymes all cost a little more than an order of magnitude more than they should. There are a few ways you can view this, but, from my perspective, it is a failure of innovation – tools of innovation like AI/ML, strain engineering, and high throughput screening work well at lowering the cost of microbial fermentation from kilograms to kilotons but begin to fail, relatively, at the small scale.
At Cocoon Bioscience, we have taken a different approach to innovation. Instead of relying on the latest and greatest technology to make the microbial fermentation process better, we opted to eliminate microbial fermentation altogether for proteins and enzymes at these small scales. Instead of using bacteria growing in a bioreactor with fixed costs that can’t magically disappear regardless of the quantity of LLMs used, we turned to nature for a better way of building a bioreactor – a cocoon. We don’t grow engineered cells in a bioreactor to produce protein – we use what nature has already evolved, the cocoon, and combine that with the system nature uses to highjack a living, multicellular organism, to make something new – a virus, or, more specifically, a virus for moths known as a baculovirus. What we found is that we can significantly and easily pull down that extended edge of the pricing curve to where it is expected to be, without sacrificing performance, quality, or speed. Actually, quite the opposite, we tend to be able to move faster and get better performance.
Scientists and engineers have squeezed an amazing amount of innovation out of microbial fermentation over the past several decades, but right now, at Cocoon, we empathize with how it must have felt like for the early scientists as they began tinkering with E.coli to produce recombinant protein in the early days of research in the field in the early 1970s – a blank canvas of possibilities exist to tackle those tough, bleeding edge problems that the current way of doing things just isn’t equipped for.
Source:
Humbird, D. (2021). Scale-up economics for cultured meat. Biotechnology and Bioengineering, 118(8), 3239-3250. https://doi.org/10.1002/bit.27848
By Cocoon Bioscience CEO, Josh Robinson